Chemistry, 9th Grade Literature & Biology
October 8, 2020
In 9th grade literature class, students worked on placing story events on the plot diagram. They had to first work to have a clear understanding of the main conflict in the story that builds the tension and moves the plot along. Then they looked back at the story to see where that conflict first appeared and also formed their interpretation of which scene was the climax of that conflict. In the photo below, they are working with a practice story, solidifying their understanding of the terms and improving their teamwork skills simultaneously.

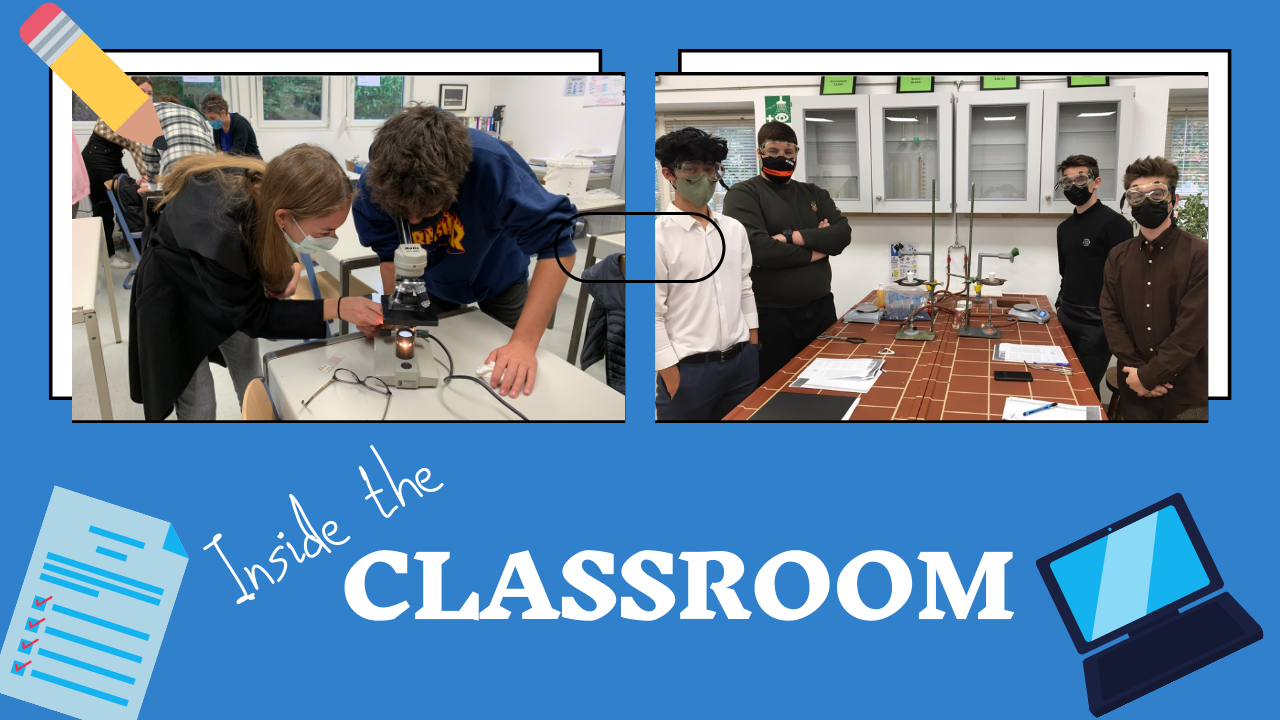
In biology class this week, our grade 9 students were learning how to use the microscope by looking for microorganisms in pond water.
In chemistry class, 11th grade students heated a hydrated copper sulfate to drive off the water of crystallization and use the mass differential to determine how many molecules of water are associated with each molecule of hydrated copper sulfate. In hydrated form, the copper sulfate forms brilliant blue crystals; in anhydrous form, copper sulfate is a white powder.




